Hyaluronic Acid
Introduction
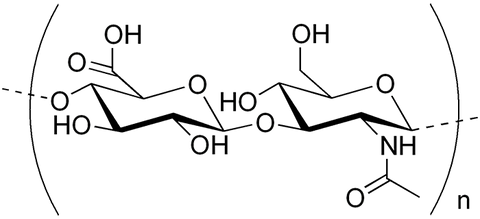
Hyaluronic Acid (or Hyaluronan) is a polysaccharide, a natural biopolymer, which belongs to the class of glycosaminoglycans. Hyaluronic Acid was discovered in 1934, when K. Meyer and J. Palmer published an article in the Journal of Biological Chemistry [1], in which they described the unusual polysaccharide isolated from the vitreous of bovine eye. At the same time, we know that even ancient Greek physicians were aware of the viscous gel, which is present in the joints, and is known today as a so-called synovial fluid or synovial liquid and consists mainly of Hyaluronic Acid.
Hyaluronic Acid is a natural compound, which is present in a human body in connective, epithelial and neural tissues. Hyaluronic Acid is a main component of extracellular matrix that can be also found in skin derma, joints synovial fluid, eyeball, hair follicles, lips, etc. Hyaluronic Acid plays tremendous role in cellular biology and has numerous biological applications such as cell nutrition, proliferation and migration, angiogenesis, apoptosis, etc. Many chemical, biological and medicinal information about Hyaluronic Acid can be found in the recent monograph [2].
The medical application of Hyaluronic Acid was started in 1950s, when Dr. E.A. Balazs initiated studies of possibility to use Hyaluronic Acid as prosthesis for the treatment of retinal detachment. Since then, Hyaluronic Acid was studied in such medicinal areas as fertilization, embryogenesis, development of the immune response, wound healing, and treatment of infectious and oncological diseases, aging process, and aesthetic medicine. Nowadays we witness an increasing interest in Hyaluronic Acid biology, which has resulted in the development of new products for pharmaceutical and aesthetic applications.
Medicinal Application of Hyaluronic Acid
Hyaluronic Acid in Aesthetic Medicine
Today, it is difficult to imagine cosmetology and aesthetic medicine without Hyaluronic Acid. Hyaluronic Acid became product #1 within this field and the sales of Hyaluronic Acid based products have recently surpassed Botox. A quick glance at world’s periodicals shows that almost every issue has at least several articles related to Hyaluronic Acid.
Initially, cosmetologists were interested in Hyaluronic Acid because of its unique ability to retain large amounts of water. This property has been maximized to its full potential for cosmetic products and moisturising masks. From the mid nineteenth to the twentieth century, chemically stabilized (crosslinked) Hyaluronic Acid was employed as the intradermal micro-implants (dermal fillers) for the contour correction of the involuntary changed skin.
The development of injection cosmetology is directly connected with the methods of contour plastic, mesotherapy, and bio-revitalization, for which an enormous number of products containing non-stabilized or partially modified Hyaluronic Acid have appeared. The cosmetologist’s toolkit was largely enhanced by a new type of products: non-medicated macromolecular therapeutic agents called “bio repairs” in which biologically active compounds are chemically immobilized on the Hyaluronic Acid macromolecules. It is important to mention that with the aid of such sustained action formulations, it is presently possible to provide target delivery of vitamins, amino acids, and oligopeptides to the specific cells, for the purposes of skin homeostasis stabilization and prevention of the age-related changes.
Hyaluronic Acid in Osteoarthritis
We know that the reducing ability of cartilage tissue chondrocytes to synthesize glucosamine is one of the main factors of osteoarthritis development. A healthy knee contains about two ml of synovial liquid and the Hyaluronic Acid concentration in it is from 2.5 to 4 mg/ml (0.25 – 0.4%). The patients with osteoarthritis have a 2-3 times lower rate of synthesis and concentration of Hyaluronic Acid in synovial liquid compared to the normal rate, reduction of the molecular weight of Hyaluronic Acid due to accelerated hydrolysis, and reduction of viscoelastic properties [3]. As a result, the friction of the surfaces of the cartilage increases as well as destruction of cartilage and bone tissue [4-6]. One of the main approach of the biomedical technology of the osteoarthritis treatment is direct injection of the products of high molecular weight or crosslinked Hyaluronic Acid. There is a significant amount of scientific, patent, and medicinal literature with positive results of the treatment of osteoarthritis by these two technologies [7-11].
Hyaluronic Acid in Ophthalmology
Hyaluronic Acid based products are widely used in both general and local therapy of eye diseases. Hyaluronic Acid is included in the composition of eye drops (“artificial tears”) for the treatment of dry corneas. Healon, a product developed by Pharmacia, Sweden, that contains high molecular weight Hyaluronic Acid, is widely used for eye surgery procedures and for surgical media (viscoprotector) to prevent the eye’s internal tissues from mechanical damages.
The vitreous body of the eye largely consists of Hyaluronic Acid produced by the cornea cells. Due to the viscoelastic properties of Hyaluronic Acid, the vitreous body assures permanent intraocular pressure and prevents detachment or retina and pigment epithelium.
Several formulations of Hyaluronic Acid solutions with amino acids, antioxidants, and other compounds have been developed. They can be prescribed for eyeball irrigation, or injection into the eye’s anterior chamber during cataract surgery [12, 13, 14].
Hyaluronic Acid in Oncology
Hyaluronic Acid is used in oncology as a therapeutic. There are various mechanisms of Hyaluronic Acid action on tumor cells. At the molecular level, the main mechanism is related to the fact that the high molecular weight Hyaluronic Acid can bind to receptors on the cell membrane of tumor cells, which results in slowed cell migration and metastasis [15]. Thus, the high molecular weight polysaccharide can be used as an inhibitor of cancer growth and metastasis formation [12]. Another mechanism of action is related to the ability of high molecular weight Hyaluronic Acid to promote the formation of a capsule surrounding the tumor. The capsule is built from the connective tissue. A third mechanism is related to the ability of high molecular weight Hyaluronic Acid to inhibit tumor vascularization (sprouting of blood vessels in the tumor), which leads to the slow growth and metastasis of tumors [16, 17]. The sprouting of the new blood vessels in the tumor (angiogenesis) is a key element of its progression. Fibroblast growth factors secreted by tumor cells stimulate proliferation of endothelial cells and promote the formation of new capillaries. Angiogenesis provides cancer cells additional benefits of growth and invasion. In this context, the researchers are faced with the duality of the physiological effect of the Hyaluronic Acid.
Another remarkable property of Hyaluronic Acid is that it enhances the effect of conventional anticancer drugs. Hyaluronic Acid allows their therapeutic doses to be reduced and consequently adverse toxicity to normal cells [18]. A large amount of Hyaluronic Acid and Hyaluronidases is found in the extracellular matrix of the tumor. Many studies demonstrated on the large number of various types of tumor cells that high molecular weight HA can inhibit both proliferation and migration of tumor cells [16-18].
The Role of Hyaluronic Acid in Healing Wounds
Hyaluronic Acid has a number of properties that favorably distinguish it from many other products used for healing wounds. Namely, Hyaluronic Acid has no allergenic nor irritational actions but can provide both anti-inflammatory and bio-stimulating effects and accelerate the regeneration processes. These properties of Hyaluronic Acid, in combination with other drugs, are used to accelerate the healing of burns, trophic ulcers, and surgical procedures. The healing of skin wounds is directly related to an increase of Hyaluronic Acid during the first three days and a decrease to the initial level by 7th day after injury. In the surgical dressing the polysaccharide is used both as a primary wound healing product, and in combination with other agents.
The bioexsplant (film) on the base of the oxidized Hyaluronic Acid demonstrated ability to accelerate healing of suture (of the intestinal high-risk anastomoses. It can also be used to close the defective serous membrane and for prophylaxis of the bowel perforation during laparoscopy [19, 20]. Hyaluronic Acid is used for the treatment of the stomach and duodenum ulcers. The anti-ulcer action of Hyaluronic Acid is related to its ability to inhibit H2 histamine receptors and the activity of trypsin [12]. Due to the ability of low molecular weight fragments of Hyaluronic Acid to penetrate the skin epidermal barrier, it is used as a transporter of bioactive compounds. According to some sources, only Hyaluronic Acid fragments with a molecular mass of 400 kDa can penetrate through the skin [21]. The smaller the fragments of the polysaccharide macromolecule the higher the permeation rate [14, 21, 22].
As mentioned above, Hyaluronic Acid is a high-rate turnover biopolymer. Its half-life in the skin is about 24 hours. Any changes in the processes of Hyaluronic Acid turnover (rate of the ratio of synthesis - decomposition) could cause development of pathologies. When a tissue is damaged, the existing Hyaluronic Acid undergoes decomposition, resulting in appearance of oligosaccharide fragments, which are involved in the formation of a temporary extracellular matrix. At the same time, the synthesis and accumulation of Hyaluronic Acid is rapidly induced in the tissue damage area by activating genes and different Hyaluronate synthases. This suggests that the macromolecule is an active participant of reparative processes of damaged tissue.
Hyaluronic Acid in Immunology
The immune system is directly connected with Hyaluronic Acid. Hyaluronan is included into drugs used for the complex treatment of immunodeficient conditions associated with viral diseases. At a molecular level, the mechanism of action of the biopolymer is connected with the blocking of several molecular inflammation factors [23]. On one hand, Hyaluronic Acid activates inferonogenesis, but on the other hand, it increases action of the interferon inductor (for example, double-stranded RNA) [24, 25]. Interferon is produced mainly by the activated monocytes and T-cells of the immune system. The interleukins-2 and -5 (IL-2, IK-5) play a major role in the activation of T-cells, which, in turn, activate synthesis of Hyaluronic Acid by endothelial capillary cells. Then Hyaluronic Acid stimulates synthesis of CD44 receptors, which is the key event for the activation of the lymphocytes and monocytes [23]. Hyaluronic Acid is used alone or in combination with interferon to slow down the development of the infection by the virus herpes simplex by application on the infected epithelium [12]. The obvious antimicrobial action of Hyaluronic Acid can be achieved by its crosslinking with hydrophilic polymers, which are capable for accelerated penetration through cell membranes or intercellular gaps [12].
Hyaluronic Acid and Anti-aging
Lately, Hyaluronic Acid products for anti-aging began attracting enormous attention. It was found that one of the most common signs of aging is reducing amount of Hyaluronic Acid in skin dermis and joints synovial fluid. Both factors play significant role in age related changes. In this context, one of the most intriguing pieces of information was published in several sources. The information is related to small Japanese village Yuzurihara. It is presented in the article Yuzurihara Reveals Its Secret: Hyaluronic Acid, “The Molecule of Youth”, written by famous Hyaluronic Acid advocate Bill Sardi.
“Late in the year 2000, ABC News Prime Time Live sent reporter Connie Chung to a small village about two hours outside of Tokyo. That report drew widespread interest. The report emanated from Yuzurihara, Japan, known as “the village of long life.” Of 990 villages and towns surveyed by the World Health Organization in Japan, there were ten times more people living beyond the age of 85 in Yuzurihara than anywhere in North America. It was so renowned, the emperor of Japan visited this village. But longevity alone was not what attracted ABC News to Yuzurihara. These aged villagers of Yuzurihara, Japan, had smooth skin, flexible joints, thick hair and few needed reading glasses. Many older residents of Yuzurihara were still farming their fields into their 80’s. These people defied their calendar age. One female resident of Yuzurihara had no wrinkles or age spots at age 90! Dr. Toyosuke Komori, the town doctor, wrote five books about Yuzurihara in the 1970’s and 80’s. He attributed the youthful aging of these people to a low-iron, sticky vegetable-based diet which ultimately promotes H.A. levels in the body. These villagers were shorter than other Japanese adults of the same age, which likely means their diet was lacking iron which is a growth factor. Dr. Komori also attributed the youthful appearance of these villagers to a molecule called Hyaluronic Acid.”
It is understandable that lately, Hyaluronic Acid as such and the products that include Hyaluronic Acid attract significant attention. At the same time, there are at least tree myths surrounding this compound.
Myths and Reality about Hyaluronic Acid
Myth # 1: Hyaluronic Acid products are only for skin and joints care
Indeed, the majority of known Hyaluronic Acid products are intended for skin and joints care. At the same time, Hyaluronic Acid was found in different organs and tissues and is known for its multiple biological applications. In addition, Hyaluronic Acid was found to possess unique ability to solubilize insoluble compounds and increase their bioavailability, as well as other biological properties. It brings the value of Hyaluronic Acid to the level, which is yet impossible to evaluate.
Myth # 2: The molecular weight of Hyaluronic Acid is unimportant
Hyaluronic Acid is not a single molecule; it is rather a family of polymeric structures with different numbers of monomers and correspondingly different molecular weights. The molecular weights could vary from several thousand up to several millions (up to 8 millions) Daltons. Each fragment has its own specific biological properties and functions; our body needs them all. There are numerous discussions regarding the value of each MW fraction in the scientific literature, however there is no consensus about which fragment absorbs better, has better value, etc. Hyaluronic Acid of high molecular weight (several hundred thousand Da) is considered excessively high to be absorbed by the body through intestine. At the same time, several scientific articles describe the results of the successful absorption of Hyaluronic Acid of million Da and higher.
Myth # 3: Hyaluronic Acid products are only for injectable or topical applications
The majority of Hyaluronic Acid products are indeed presented on the market as injectable products for skin and joints, as well as cosmetic products (serums, creams and lotions) for topical applications. It was believed that upon oral administration, Hyaluronic Acid is being quickly hydrolyzed in the stomach, which eliminates any positive effect. However, a number of recent scientific publications have revealed the results of clinical studies demonstrating that Hyaluronic Acid administrated orally can be quantitatively delivered to skin and joints. Hyaluronic Acid is quite easily absorbed in the intestines by different mechanisms and deliver itself (as well as the products attached to Hyaluronic Acid) to various organs and tissues. Many other questions related to Hyaluronic Acid biology are addressed in the monograph [2].
Orally Administrated Hyaluronic Acid Products
Since the possibility of orally delivered Hyaluronic Acid products became a reality, several scientific studies have been recently published. These studies revealed research data, which open many doors to application of Hyaluronic Acid products in different therapeutic areas.
Hyaluronic Acid: Animal Studies
In 2008 a study conducted on rats and dogs determined the absorption, distribution and excretion of high-molecular-weight Hyaluronic Acid radiolabeled with two isotopes of technetium, after single dose, oral administration. Autoradiography of skin, bone and joint tissue pieces after 24 h showed incorporation of radioactivity, although different isotopes absorbed differently. Overall, the study showed an expected pattern of rapid absorption and excretion in urine, with accumulation in thyroid glands, stomach, kidney and bladder. This report presents the first evidence for uptake and distribution to connective tissues of orally administered, high-molecular weight Hyaluronic Acid [26].
Another study carried out on male rats, which orally received 14C-labeled Hyaluronic Acid. The study results showed that after 8 hours after administration, 14C-Hyaluronic Acid was found in the blood. Approximately 90% of 14C-Hyaluronic Acid was absorbed from the digestive tract and used as an energy source or a structural constituent of tissues [27].
Hyaluronic Acid: Clinical Studies
In 2012, Japanese researchers reported the results of the clinical study, in which the effect of orally administrated Hyaluronic Acid on the knees osteoarthritis had been studied. The double-bind placebo-controlled study over 12-month period showed that daily intact of 200 mg of orally administrated Hyaluronic Acid alleviated the symptoms of knee osteoarthritis in patients aged 70 years or younger [28].
Another human study was designed to determine clinical effect of dietary Hyaluronic Acid on dry, rough skin. Double-blind feeding study was carried out on 35 subjects, who frequently suffer from dry, rough skin. The daily intake was 120 mg/day of Hyaluronic Acid for 4-weeks period. Two skin parameters were measured during the study – measurements of skin moisturising, which is result of injected Hyaluronic Acid and skin smoothness and ameliorates wrinkles. The study results showed that ingestion of Hyaluronic Acid is effective at increasing moisture retention and smoothness in the skin, and there are no safety concerns [29].
The same group of scientists conducted comprehensive research in 2014, in which they studied effect of oral Hyaluronic Acid for the dryness of skin, induced by aging, UV radiation smoking and air pollutants. They found that daily Hyaluronic Acid supplements can moisturize the skin because the metabolites of Hyaluronic Acid increase the skin moisture content by having the effect on the ski cells. Thus, consuming Hyaluronic Acid affects skin and improves dry skin physiologically [30].
A recent study conducted by Italian scientists connected excessive exposure to the sun with severe photoaging, which resulted in a loss of physiological elastic fiber functions. The study results concluded that the complex dietary supplement, which contained Hyaluronic Acid, resulted in an improved VAS photoaging score, if compared with placebo group. Such results were observed even 2 weeks after treatment [31].
As mentioned above, second major application of Hyaluronic Acid products is a treatment of joints pain and other complications of osteoarthritis. The comprehensive review, which gives excellent summary of the different products and their effect after treatment was recently published [32]. The review summarizes the data of 13 human clinical studies and 28 safety studies, both human and animal. The studies results included such statements as significant improve of knee pain and discomfort, significant improve of knee pain and stiffness, significant improve of synovial effusion and knee pain, significant improve of pain/step-up and -down function and aggregate total symptoms, significant improve of joint mechanics and muscle function, significant improve of muscle function, synovial effusion and reduces pain, significant improve of articular pain, synovial effusion and knee muscular strength [32].
In the light of discussion of possibility of absorption of high MW hyaluronic acid, it is interesting to discuss the results of the study, mentioned in the review [32]. The study evaluated the effects of daily oral intake of a consumable liquid fermentate containing high-molecular-weight Hyaluronic Acid, ranged between 2.5 and 2.8 million Daltons. A basic evaluation of safety and tolerability was performed as well. A randomized, double-blind placebo-controlled study design was used to examine the effects of oral intake of Hyaluronic Acid on chronic pain conditions. Safety assessment included a complete blood count with differential, blood chemistry and electrocardiogram. The results clearly showed that consumption of an oral liquid formula containing high molecular-weight Hyaluronic Acid was associated with relief of chronic pain [33].
Based upon recent changes in recommended osteoarthritis management, where injectable Hyaluronic Acid is no longer recommended [34], alternative methods for managing chronic joint pain are in high demand. The data presented in recent studies of oral hyaluronic acid suggest that high-molecular-weight Hyaluronic Acid offers a non-invasive method for pain management in situations involving moderate chronic joint pain affecting mobility.
As a conclusion, we would like to once again refer to the above mentioned article by Bill Sardi: “Oral supplementation with Hyaluronic Acid is useful in maintaining skin elasticity, joint flexibility and sharp vision among adults. For the first time, an oral, non-prescription substance is available that supports youthful appearance, joint movement and visual response among adults of a wide age range”.
REFERENCES
1. Meyer, K., Palmer, J. (1934) The polysaccharide of the vitreous humor. Journal of Biological Chemistry, 107, 629-634.
2. Selyanin, M.A, Boykov, P.Ya, Khabarov, V.N., (ed. F. Polyak) (2015) Hyaluronic Acid: Preparation, Properties, Application in Biology and Medicine. John Wiley & Sons, Chichister, West Sussex, UK.
3. Balazs, E.A., Watson, D., Duff, J.F., Roseman S. (1967) Hyaluronic acid in synovial fluid. 1. Molecular parameters of hyaluronic acid in normal and arthritis human fluids. Arthritis & Rheumatism, 10, 357-376.
4. Matveeva, E.L. (2007) Study of age-related changes and gender features of the biochemical composition of human knee joint synovial fluid (in Russian). Klinicheskaya Laboratornaia Diagnostika, (5), 15-17.
5. Vo, N., Niedernhofer, L.J., Nasto, L.A. et al. (2013) An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. Journal of Orthopaedic Research, 31(6), 831-837.
6. Lee, A.S., Ellman, M.B., Yan, D. (2013) A current review of molecular mechanisms regarding osteoarthritis and pain. Gene, 527 (2), 440-447. 56
7. Vallières M., du Souich P. (2010) Modulation of inflammation by chondroitin sulfate. Osteoarthritis Cartilage, Suppl. 1:S1-S6.
8. Monfort J., Pelletier J.P., Garcia-Giralt N., Martel-Pelletier J. (2008) Biochemical basis of the effect of chondroitin sulphate on osteoarthritis articular tissues. Annals of Rheumatic Diseases, 67 (6), 735-740.
9. Im, G.I., Choi, Y.J. (2013) Epigenetics in osteoarthritis and its implication for future therapeutics. Expert Opinion on Biological Therapy, 13 (5), 713-721
10. Goldberg, V.M., Buckwalter, J.A. (2005) Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage, 13 (3), 216-224
11. Dougados, M. (2000) Sodium hyaluronan therapy in osteoarthritis: Arguments for a potential beneficial structural effect. Seminars in Arthritis and Rheumatism, 30 (2), 19- 25.
12. Radaeva, I.F., Kostina G.A. (1998) Use of hyaluronic acid for the treatment of various pathologic states. Pharmaceutical Chemistry Journal, 32 (9), 492-494.
13. Goa, K.L., Benfield, P. (1994) Hyaluronic acid. A review of its pharmacology and use as a surgical aid in ophthalmology and its therapeutic potential in joint disease and wound healing. Drugs, 47 (3), 536-560.
14. Peck, C.M, Joos, Z.P., Zaugg, B.E. et al. (2009) Comparison of the corneal endothelial protective effects of Healon-D and Viscoat. Clinical & Experimental Ophthalmology, 37 (4), 397-401.
15. Austa, L., Clark, C., Turle, E.A.,Vandelig, K. (1991) Hyaluronan and cell-associated hyaluronan binding protein regulate the locomotion of ras-transformed cell. Journal of Cellular Biology, 112 (5), 1041-1047.
16. Rooney, P., Kumar, S., Ponting, J., Wang, M. (1995) The role of hyaluronan in tumor neovascularization. International Journal of Cancer, 60, 632-636.
17. Delpech, B., Girard, N., Bertrand, P., Courel, M.N. (1997) Hyaluronan: fundamental principles and applications in cancer. Journal of Internal Medicine, 7, 41-48.
18. Filion, M.C., Menard, S., Filion, B., et al. (2002) Anti-cancer Activity of Hyaluronan, in Hyaluronan: Proceedings of an International Meeting, September 2000, North East Wales Institute, UK (eds. J.F. Kennedy, G.O. Phillips, P.A. Williams, V.C. Hascall), Woodhead Publishing Ltd., Cambridge, 419-427.
19. Ibragimov, R.M., Khasanov, A.G., Kayumov, F.A., Sufiyarov I.F. (2010) Prophylaxis of incompetence of hollow organs anastomoses by hyaluronic acid-based bioexplant (in Russian). Rossiiskie Medicinskie Vesti, 15 (2), 45-50.
20. Chernousov, A.F., Khorobrykh, T.V., Antonov O.N. (2005) Prophylaxis of insufficiency of gastrointestinal anastomoses (in Russian) Khirurgiya, (12), 25-29.
21. Brown, T.J., Alcorn, D., Fraser, J.R. (1999) Absorbtion of hyaluronan applied to the surface of intact skin. Journal of Investigative Dermatology, 113 (5), 740-746.
22. Maytin, E.V., Chung, H.H., Seetharaman, V.M. (2004) Hyaluronan participates in the epidermal response to disruption of the permeability barrier in vivo. American Journal of Phathology, 165, (4), 1331-1338.
23. Pure, E., Сulf, C.A. (2001) A crucial role for CD44 in inflammation. Trends in Molecular Medicine, (7), 213-221.
24. Shkil', N.N., Glotov, A.G., Glotova, T.I. (2003) Influence of hyaluronic acid gel on interferonogenesis in mice (in Russian). Voprosy Virusologii, 48 (5), 26-29.
25. Barinskii, I.F., Alimbarova L.M., Samoilenko I.I. (1988) Development of suppository form of interferon inductor `poludan` and potentiation of its activity by hyaluronic acid (in Russian). Voprosy Virusologii, 10, 237-239.
26. Balogh, L., Polyak, A., Mathe, D., Kiraly, R., Thuroczy, J., Terez, M., Janoki, G., Ting, Ya., Bucci, L.R., and Schauss, A.G. (2008) Absorption, Uptake and Tissue Affinity of High-Molecular-Weight Hyaluronan after Oral Administration in Rats and Dogs. J. Agric. Food Chem. 56, 10582-10593.
27. Oe, M., Mitsugi, K., Odanaka, W., Yoshida, H., Matsuoka, R., Seino, S., Kanemitsu, T., and Masuda, Ya.(2014) Dietary Hyaluronic Acid Migrates into the Skin of Rats. Scientific World Journal, 2014, 1-8.
28. Tashiro, T., Seino, S., Sato, T., Matsuoka, R., Masuda, Ya., Fukui, N. (2012) Oral Administration of Polymer Hyaluronic Acid Alleviates Symptoms of Knee Osteoarthritis: A Double-Blind, Placebo-Controlled Study over a 12-Month Period. Scientific World Journal. 2012, 1-8.
29. Sato T., Sakamoto W., Odanaka W., Yoshida K., Urishibata O. (2002) Clinical effect of dietary Hyaluronic Acid on dry, rough skin. Aestetic Dermatology, 12, 109-120.
30. Kawada Ch., Yoshida T., Yoshida H., Matsuoka R., Sakamoto W., Odanaka W., Sato T., Yamasaki T., Kanemitsu T., Masuda Ya, Urushibata O. (2014) Ingested hyaluronan moisturizes dry skin. Nutrition Journal. 13, 70-78.
31. Di Serbo A., Laurino C., Palmieri B., Lannitti T. (2015) A dietary supplement improves facial photoaging and skin sebum, hydration and tonicity modulating serum fibronectin, neutrophil elastase 2, Hyaluronic Acid and carbonylated proteins. Journal of Photochemistry and Photobiology, 144, 94-103.
32. Oe, M., Tashiro, T., Yoshida, H., Nishiyama, H., Masuda, Ya., Maruyama, K., Koikeda, T., Maruya, R. and Fukui, N. (2016) Oral hyaluronan relieves knee pain: a review. Nutrition Journal 15:11, 1-15.
33. Jensen G.S., Attridge V.L., Miki R. Lenninger M.R., Kathleen F. Benson K.F. (2015) Oral Intake of a Liquid High-Molecular-Weight Hyaluronan Associated with Relief of Chronic Pain and Reduced Use of Pain Medication: Results of a Randomized, Placebo-Controlled Double-Blind Pilot Study J Med Food, 18, 95-101.
34. Jevsevar, D.S. (2013) Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg 21, 571-576.

